Image with permission http://www.wopc.co.uk/
A Jack of all Trades in Cancer Cells
By Thomas Arnesen and first published on UiB.no March 14, 2019.
For Norwegian version click here.
Cancer is a complex disease caused by a multitude of factors gone wrong in the cell. NAA10 may be one such factor. This is a protein that performs many different tasks, including the most common which is catalyzing the acetylation of cellular proteins. NAA10 can therefore be viewed as a ”Jack of all trades”-protein. For two decades it has been linked to cancer progression, but recent data puts this enzyme on the cancer map for real. NAA10 displays a surprising variety of biochemical functions which direct many cancer signaling pathways and is now emerging as a major regulator of apoptosis, autophagy and metastasis.
Investigations twenty years ago at Haukeland University Hospital revealed a novel gene upregulated in aggressive thyroid cancers (Fluge et al., Oncogene, 2002). This gene encodes the NAA15 protein which was found to be the first member of a novel human enzyme family, the protein N-terminal acetyltransferases (NATs) (Arnesen et al., Biochem J, 2005). NAA15 complexes with NAA10 to form NatA which is essential for cancer cell survival by inhibiting apoptosis (Arnesen et al., Oncogene, 2006). NatA acetylates the N-terminal end of around 40% of all human proteins (Arnesen et al., PNAS USA, 2009) and thereby creates a potential for protein regulation.
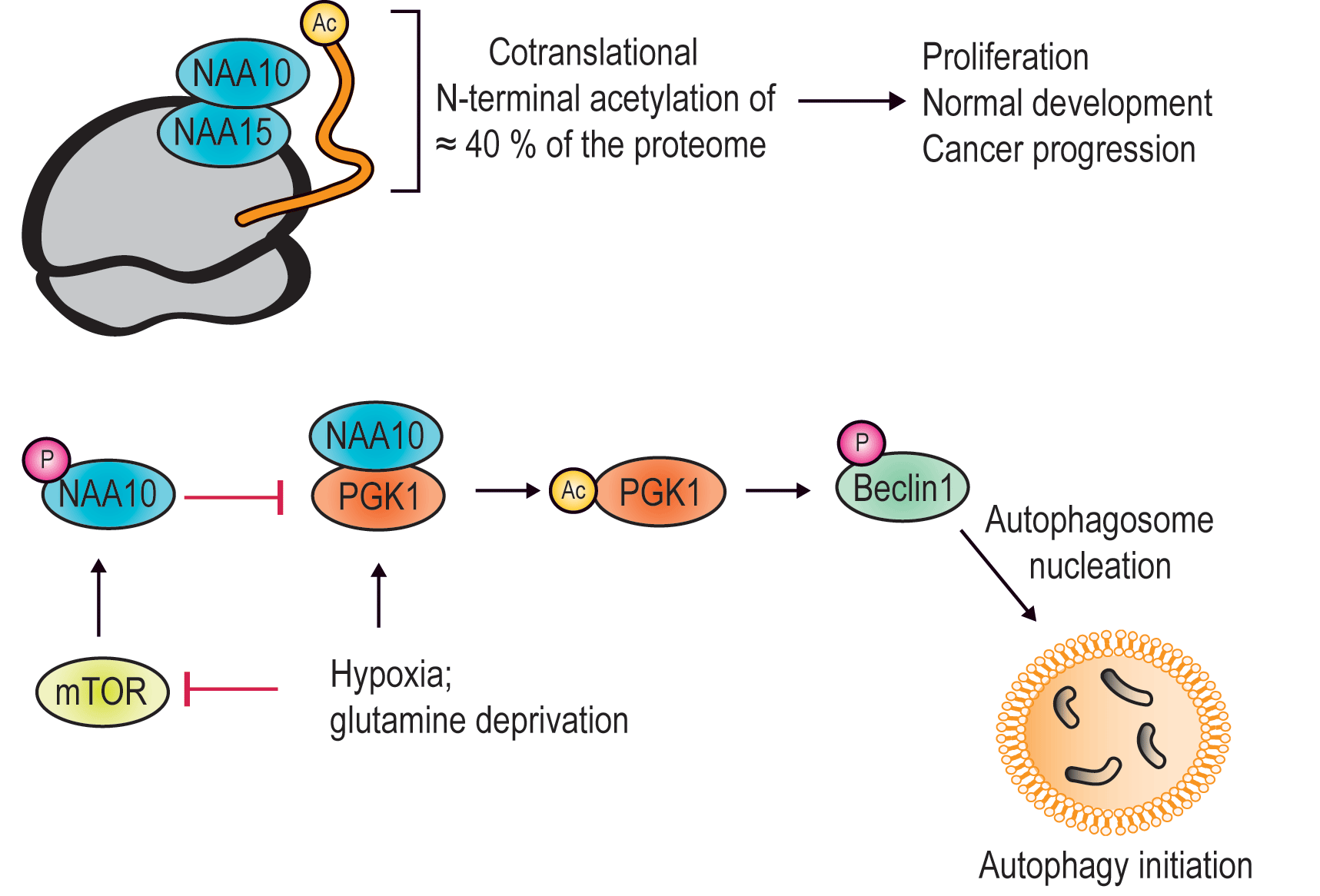
Top: NAA10 in complex with NAA15 is an N-terminal acetyltransferase (NAT) acetylating 40% of our proteins when they are produced at the ribosome. Bottom: NAA10 is a lysine acetyltransferase (KAT) acetylating among others PGK1 and thereby initiates formation of autophagosomes.
Image: Aksnes, Ree and Arnesen
A similar enzyme family, lysine acetyltransferases (KATs), perform another type of protein acetylation, namely acetylation of lysine side chains of proteins and is well-known for its impact on gene regulation and epigenetics (histone acetylation). Classically, we think that one protein has one specific role in our cells, but several proteins with multiple functions, or so called moonlighting proteins are known to exist. Regarding protein acetylation, NAA10 appears to be unique in its capacity to catalyze acetylation of lysine residues of proteins in addition to N-terminal ends, depending on context. This way, it acetylates and regulates several key proteins in different ways. These findings and other developments in the field are now published by Henriette Aksnes, Rasmus Ree and Thomas Arnesen at the Department of Biomedicine, UiB (Aksnes et al., Mol Cell, 2019). First, the oncoprotein Beta-catenin (Lim et al., Cancer Res, 2006) is acetylated and activated by NAA10 leading to cell proliferation. A second target is the master regulator of a cancer cell’s response to hypoxia, the transcription factor Hypoxia inducible factor 1alpha (HIF-1α) (Kang et al., Redox Biol, 2018), and the acetylation mediates its degradation under normal oxygen levels. Third, Heat shock protein 70 (Hsp70) works to promote protein refolding after chemical stress, and NAA10 acetylation of Hsp70 promotes its refolding activity (Seo et al., Nature Commun, 2016). A fourth target is androgen receptor (AR) and NAA10 mediated acetylation of AR promotes the expression of AR target genes in prostate cancer (Wang et al., PNAS USA, 2012). A final example is phosphoglycerate kinase 1 (PGK1). During normoxia, NAA10 is phosphorylated by the famous kinase mammalian target of rapamycin (mTOR), which blocks its interaction with PGK1. Hypoxia or glutamine deprivation inhibits mTOR so that NAA10 is allowed to acetylate PGK1, which in turn may phosphorylate Beclin1 and start the process of autophagosome nucleation. NAA10 thus regulates nutrient availability and macromolecule recirculation through induction of autophagy under hypoxic or stress conditions (Qian et al., Mol Cell, 2017).
As if this duality was not enough, NAA10 also controls cancer cells independent of its acetyltransferase activities. Two of its non-catalytic interactions impact the metastatic potential of cancer cells. In the first case, NAA10 interaction with STAT5a decreases the expression of STAT5a target genes like the pro-metastatic ID1 (Zeng et al., Carcinogenesis, 2014). NAA10 also binds to PIX and thereby disrupts PIX’s binding to GIT thus reducing Cdc42 and Rac1 activity at focal adhesions and decreasing the rate of cell migration (Hua et al., Mol Cell, 2011). Thus, in both these cases, NAA10 appears to have an anti-metastatic function.
So while displaying strong oncoprotein features in primary tumors as an acetyltransferase, its anti-metastatic capacity as a non-catalytic regulator would suggest that drugs specifically inhibiting its acetyltransferase activity while maintaining or increasing its non-catalytic mode could be highly beneficial for cancer treatment.
Co-translational, Post-translational, and Non-catalytic Roles of N-Terminal Acetyltransferases
Henriette Aksnes, Rasmus Ree, and Thomas Arnesen
Mol Cell. 2019 73(6):1097-1114